How to fix male sperm
Some men have either defective sperm genes and can't make sperm (some conditions known as azoospermia), or have defective genes that affect other bodily functions. Until the 21st century, there was nothing men could do - they had to live with the inability to produce sperm, or while living with a disease caused by a defective gene, having the fear of passing that gene onto their children (and there are some reallllllly nasty genetic diseases). Religious people might argue - tough luck that is God's will - but scientists, thinking about similar objections against giving women anesthetic during childbirth, argue "if we can fix it, let's fix it".
Recounting from the making male sperm Web page, making male sperm is a six step process - CREATE, LOCATE, COOK, SIMMER, DAZZINGLY SPLIT and ASSEMBLE. In the 1990s, scientists at the University of Pennsylvania, lead by Professors Ralph Brinster and James Zimmermann, invented a biotech method to fix genetic defects in male sperm. In their method, discussed on this Web page, they changed the fifth step of making male sperm, the DAZZINGLY SPLIT step, by adding a four act introduction: EXTRACT or CLONE, ZAP, FIX, REPLANT, so that making fixed male sperm is as follows:- CREATE
- LOCATE
- COOK
- SIMMER
- EXTRACT - extract germ cells from the testicles
- ZAP - kill the remaining germ cells in the testicles
- FIX - fix the genes in the extracted germ cells
- REPLANT - inject the fixed germ cells back into the testicles
- CLONE - if no germ cells to extract, clone them
- DAZZINGLY SPLIT
- ASSEMBLE
Such techniques were and are controversial. Indeed, when Brinster's and Zimmermann's work became publicly known thanks to an April 1994 article in New Scientist, there was an uproar around the world. Interestingly, despite the scrutiny given to their work in 1994, no one bothered to closely read their patent application and notice a suggestion made with regards to female sperm ("no one" including the Bioethics Center at the University of Pennsylvania where Brinster and Zimmermann were doing their work).

Click here for Page 1 and Page 2 of the original article. In the years to follow, other labs confirmed Brinster's results with mice, including demonstrating that replanted germ cells that were transformed into sperm can be successfully used to fertilize eggs.
EXTRACT - extract germ cells from the testicles
The first step is to extract some germ cells from the testicles of an adult male, preferably from the seminiferous tubules [needle (1) in the diagram below].
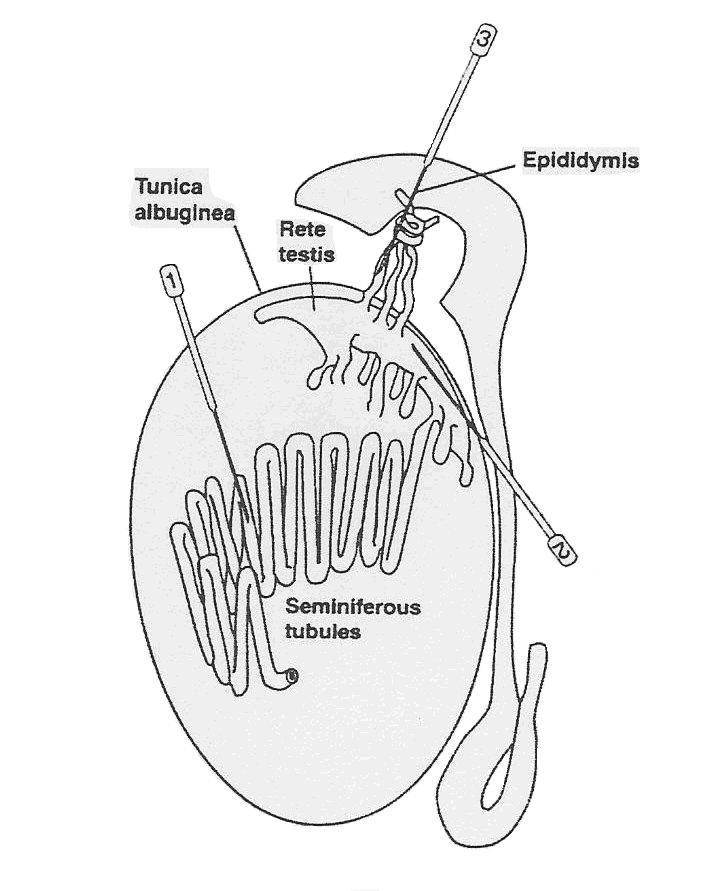
Once extracted, the germ cells can be expanded in vitro.
ZAP - kill the remaining germ cells in the testicles
Before the fixed germ cells are placed back in the testicles, all other germ cells in the testicles have to be destroyed without affecting the cellular structures (Sertoli cells, Leydig cells ???) that are needed to make sperm. Fortunately, there are safe methods for removing these remaining germ cells. Again, from Brinster's and Zimmermann's 1991 patent application:
A male host animal is prepared by destroying the native germ cell population in the seminiferous tubules by a method that leaves intact (i.e. biologically functional) the supporting cells, including the Sertoli cells. Suitable known methods include physical means (e.g. radiation, heat, etc.), chemical means (e.g. cadmium, Busulfan, etc.), but any other known techniques to selectively destroy native sperm cells can be used.
Preferably radiation is used, with Busulfan being most preferred. A typical Busulfan treatment is illustrated as follows. Forty milligrams of Busulfan are dissolved in 10 millileters of dimethylsulfoxide, to which 10 millileters of water are then added. An aliquot of this solution is injected intra-peritoneally into a mouse sufficient to administer a dose of 1 milligram of Busulfan per 25 grams of mouse weight.
Busulfan is a drug used in chemotherapy since it targets dividing cells (and cancer cells do love to divide, as do germ cells).

FIX - fix the genes in the extracted germ cells
As Brinster and Zimmermann point out in their patent, the extracted germ cells can be repaired before they are injected back into the testicles. Again, from Brinster's and Zimmermann's 1991 patent application:
In an optional embodiment of the invention, the primitive cells are modified genetically by a variety of known techniques so that the genetic characteristics of the resulting spermatozoa are predetermined.
[..........]
The present invention also has applications in gene therapy, including human gene therapy. For example, a patient with a deleterious genetic trait could undergo a testicular biopsy. Isolated stem cells can be genetically modified to correct the deleterious trait. The patient then undergoes a treatment to remove the remaining germ cells from his testes, for example by specific irradiation of the testes. His testes (now devoid of germ cells) can then be recolonized by his own, genetically corrected, stem cells. The patient can then father progeny free from the worry that he would pass on a genetic disease to his progeny.
REPLANT - inject the fixed germ cells back into the testicles
Finally, we inject the germ cells back into the testicles of the male from whom the germ cells were extracted (or another male who volunteers the use of his testicles - the testis is immune-privileged), preferably into the seminiferous tubules [needle (1) in the diagram below. Once injected, the altered germ cells go through the processes of spermatogenesis and spermiogenesis to have sperm created, in particular, with the germ cells being paternally imprinted.
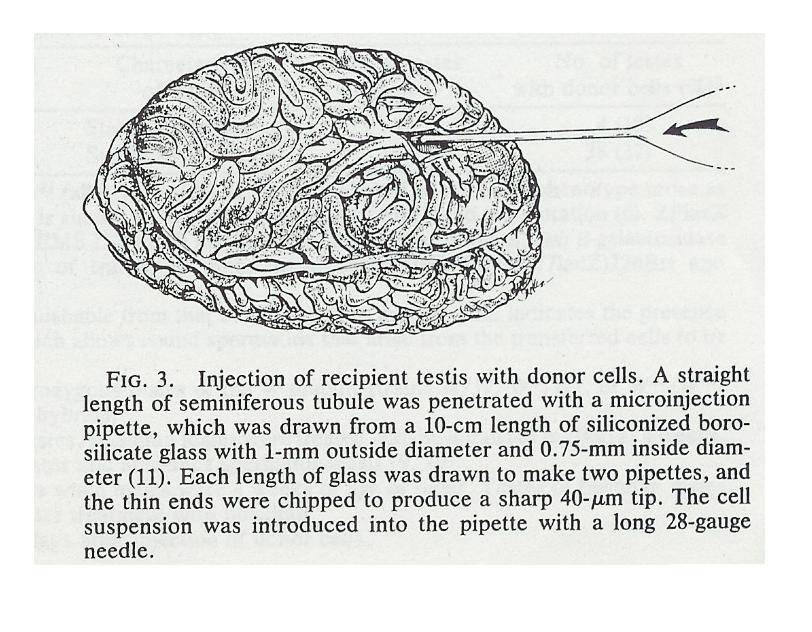

As Brinster point out, you can use another male to inject the germ cells into, make like women who make their wombs available. Indeed, you might not even need a male of the same species. For example, Brinster has been able to grow rat sperm in mouse testicles, even though mouse sperm and rat sperm take different amounts of time to grow.. He was not as successful growing germ cells in mouse testis cells for less related animals such as dogs, cows, pigs, primates. Rat and mouse testis cells have much in common since rats and mice diverged genetically only about 10 million years ago, while the other animals diverged genetically over 50 million years ago, and therefore their testis cells have less in common.
However, 10 millions years is on the order of when humans diverged genetically from chimpanzees and gorillas (humans and chimpanzees diverged about 6-8 million years ago), making the latter potential hosts for growing human sperm, if the supporting cells for growing sperm (Sertoli, etc.) have a lesser role than the germ cells. As Brinster puts it in a 21 June 2002 paper in Science titled "Germline stem cell transplantation and transgenesis":
The environment required for stem cell and undifferentiated spermatogonial cell maintenance appears to be highly conserved, whereas factors necessary for the differentiation process diverge more quickly.
Thus the rigid species timing and pattern of germ cell differentiation reflects the intrinsic genetic program of the germ cell and is not influenced by somatic supporting cells.
I can't see the FDA ever approving of this in practice.
CLONE - if no germ cells to extract, clone them
Now Brinster's work assumed, even for males with defective sperm making genes, that the male still has at least some adult germ cells in his testicles. But how do you help a male with no adult germ cells in his testicles? How can you recreate his germ cells? Clone him to recreate his original yolk sac and migrating primordial germ cells.
Cloning humans is still in its infancy, and will require decades of research (mostly done outside the United States) until cloned cells can be routinely used for therapeutic purposes, or in this case, providing a source of female germ cells for creating female sperm. One difficulty with human cloning is resetting the imprinting of chromosomes in the cell nucleus being transplanted into an empty egg.
Extracting migrating germ cellsAs you recall, germ cells appear in the yolk sac of the embryo before migrating to the developing genital region of the embryo. An extremely important question arises, - "When do you extract germ cells from the cloned embryo?", more specifically, "When do you extract germ cells from the cloned embryo, germ cells that have had their imprinting erased?". As you recall, some genes are imprinted to indicate that they came either from a male or female. During the earlier stages of an embryo, all of the parental imprinting is erased (though there are some reports of grandparent imprinting effects) so that the embryo, once it determines it sex, can mark its sperm or eggs appropriately.
Research indicates that the timing of this erasure is early in the life of the embryo. A paper in the 14 October 2003 issue of PNAS, titled "Reprogramming of promordial germ cells begins before migration into the genital ridge, making these cells inadequate donors for reproductive cloning", reports on results on studies on mouse embryos. Here a group of researchers lead by a team at the University of Pennsylvania, found that at 13.5 days post-coitum (dpc), the imprinting was completely erased for the germ cells found on the genital ridges, with imprinting starting a few days later.
Given similarities between humans and mice, there should be a time when human germ cells have their imprinting erased. By extracting the germ cells from the clone, one has created germ cells ready to be transformed (the FIX stage above) and then transplanted into the testicles (the REPLANT stage above) to be transformed into sperm with genes paternally imprinted.
The extracted germ cells can also be increased in number by growing them in culture.
Using embryonic stem cells from blastocystsExtracting germ cells requires the embyro to grow for about two months. While this is still within the first trimester where processes like abortion are allowed, it is preferable to make use of stem cells acquired closer to fertilization. In 2003, experiments were performed that showed that embryonic stem cells can produce functional germ cells in vitro, germ cells that can be used for fertilization. Such embryonic stem cells were extracted from blastocysts about five days after fertilization, about the same time cloned stem cells will be extracted for therapeutic purposes. Embryonic stem cells are also amenable to genetic alteration.
The following diagram illustrates this process for generating neural and pancreas cells from embryonic stem cells.
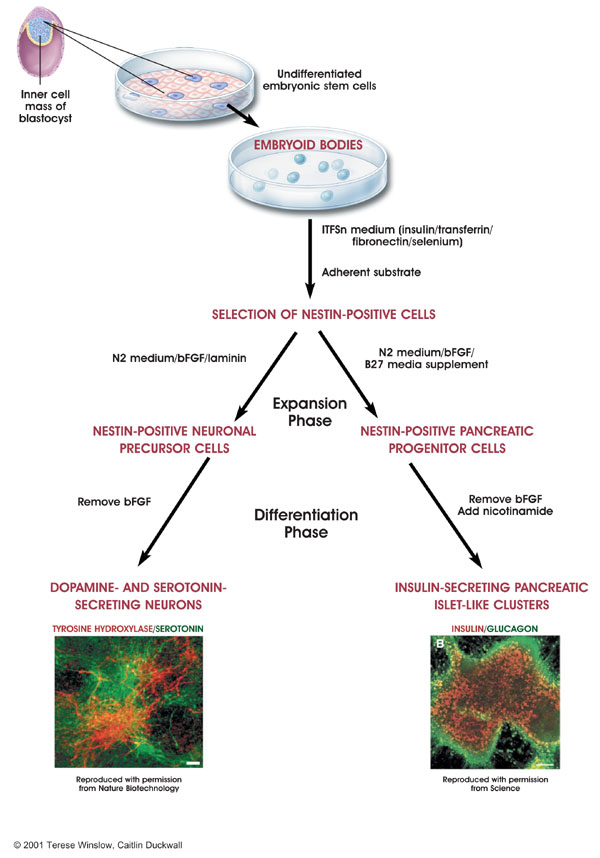
Picture credit:
National Institute of Health
Not surprisingly, cloning technologies are being patented, allowing the patent owner to control the exploitation of the technology. Click here for a nice introduction to patents on mammalian cloning provided by patent lawyer Gerry Elman.